Discover Related Products
Now explore supplements designed to support Longevity, Gut Health & Cellular Vitality.
CITOZYM - Support Natural Detox, Immune and Longevity Support
Regenerative Mechanisms in Animal Models: An In-Depth Analysis
Introduction
Regeneration, the ability to restore damaged or lost body parts, represents one of the most fascinating and complex phenomena in biology. While many organisms, including mammals, display limited regenerative capabilities, certain species have developed highly specialized mechanisms to regenerate limbs, tissues, and even entire body parts. Understanding how these organisms coordinate tissue repair offers unique insights into potential therapeutic approaches for human regenerative medicine. Among the most studied models are planarians, Hydra, axolotls, lobsters, and various insects, each of which showcases unique strategies for regeneration.
Planarians: Regeneration and the Role of Neoblasts
Planarians, particularly Schmidtea mediterranea, are flatworms known for their extraordinary ability to regenerate any part of their body thanks to a population of adult stem cells called neoblasts. These are the only mitotically active cells in the organism, distributed throughout the body except for certain regions. Regeneration in planarians is initiated by two distinct mitotic peaks: an initial systemic peak that responds to any injury and a localized peak that occurs specifically when tissue loss is involved [1].
The regenerative capacity of planarians relies heavily on the migration and proliferation of neoblasts toward the wound site, where they form an aggregate of undifferentiated cells known as a blastema. Under the influence of local and systemic molecular signals, the blastema differentiates to replace lost tissues. This process not only reconstructs anatomical structures but also facilitates the functional regeneration of complex organs, such as the central nervous system. The regulation of neoblast behavior involves complex interactions between wound signals, tissue polarity, and gradients of regulatory molecules that precisely guide tissue formation [1].

Fig. 1 Model of the planarian neoblast wound response. Cartoon summarizing cellular changes during regeneration initiation. Two distinct phases of neoblast responses occur during regeneration initiation. The first phase represents a generic response to injury that spreads quickly from the wound site, is wound-size dependent, and induces increased mitotic entry of neoblasts body-wide. The second phase occurs only when a significant amount of tissue is missing and involves local signals that induce recruitment of neoblasts followed by local proliferation and differentiation at the wound site. Depicted are posterior (tail) fragments [1].
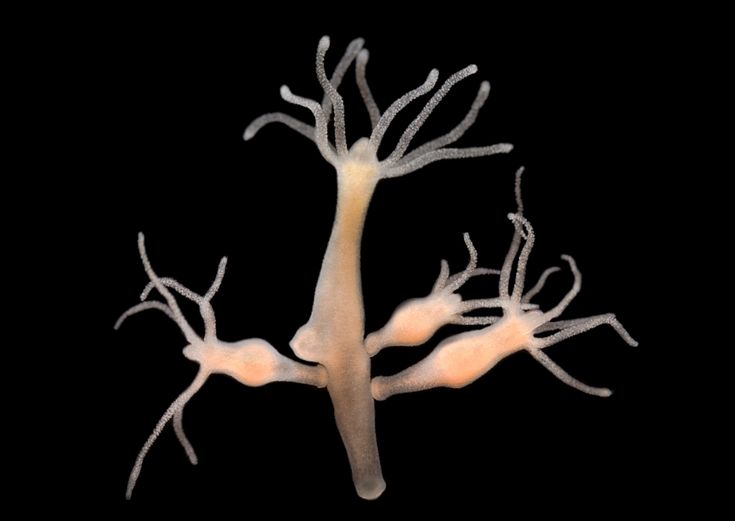
Hydra: Whole-Body Regeneration and Patterning Control
Hydra, a small freshwater polyp, can regenerate its entire body from tissue fragments due to three main populations of stem cells: epithelial epidermal cells, epithelial gastrodermal cells, and multipotent interstitial cells. Regeneration in Hydra is governed by an organizer center that coordinates growth and patterning of new tissues through long-range signals. This process involves the activation and differentiation of stem cells, which organize to form complex structures like the organism’s head and foot [2].

Fig. 2 Phylogenetic position and regenerative capabilities of Hydra. (A) Phylogenetic position of Hydra within the phylum Cnidaria and the class Hydrozoa. (B) Hydra anatomy. On their apical end, animals possess a head consisting of the hypostome and tentacles. The body column separates the head from the foot, which is located at the basal end. (C) Haematoxylin and Eosin staining of paraffin embedded sections through a Hydra animal, highlighting the two distinct body layers (the epidermis and the gastrodermis) and the ECM layer (the mesoglea) that separates them. (D) Hydra head and foot regeneration. Regenerating animals after mid-gastric bisection at the indicated time points are shown. Blue arrow indicates the fully regenerated foot. Green arrows indicate the emergence of tentacle rudiments. Red arrow indicates a fully regenerated head. Scale bars: 500 μm in B,D; 20 μm in C [2].
Hydra uses a self-organizing system to ensure structural and functional integrity during regeneration. The biochemical and mechanical signals that regulate this process have been extensively studied, revealing how their balance is crucial for the correct development of new structures. The ability to genetically manipulate Hydra, using techniques such as transgenesis, has helped identify the genes and signaling pathways that control regeneration, providing valuable insights into how cellular signals are coordinated to promote regeneration [2].

Fig. 3 Regeneration of Hydra from reaggregated cells. The reaggregation experiment (top panel) was made with Hydra taken from two distinct transgenic AEP strains: one that expresses eGFP under the control of the actin promoter in epidermal cells; and the other that expresses RFP under the control of the actin promoter in gastrodermal cells. Aggregates were imaged as indicated at different time points after re- aggregation (lower panels). The re-aggregated cells are sorted, with gastrodermal cells (red) located inside the aggregate and epidermal cells (green) in the periphery, and there is a subsequent regeneration of a complete Hydra animal. Scale bar: 250 μm [2].
Lobsters: Telomerase Activity and Indeterminate Growth
Lobsters (Homarus americanus) represent a unique case among regenerative organisms due to their indeterminate growth and high telomerase activity, present in all tissues. Telomerase is an enzyme that adds repetitive sequences to telomeres, protecting cells from progressive shortening and ensuring continuous proliferation. In mammals, telomerase activity is often restricted to specific cells, such as embryonic stem cells or cancer cells; however, lobsters maintain ubiquitous activity, allowing them to continue growing and regenerating limbs even at advanced ages [3].
This characteristic suggests that telomere length regulation and telomerase activation may play crucial roles in regeneration and longevity. The absence of evident cellular senescence in lobsters highlights the potential role of these mechanisms in preventing aging and enhancing regenerative capacity. Studies on this model could contribute to the development of therapies aimed at extending human lifespan and improving tissue regeneration [3].
Axolotls: Limb Regeneration in Vertebrates
The axolotl (Ambystoma mexicanum) is one of the few vertebrates capable of regenerating complete limbs after amputation. Regeneration occurs through the formation of a blastema, an aggregate of mesenchymal cells that dedifferentiate and proliferate to rebuild the lost tissue. Blastema cells originate from various tissues, including muscle, skin, and bone, and undergo reprogramming to revert to an embryonic-like state. The regenerative process is orchestrated by complex signaling networks, including WNT, FGF, and neuregulin pathways, which control cell proliferation, blastema maintenance, and fate specification [4] [5].

Fig. 4 Principles of Limb Regeneration Revealed by Classical Grafting Experiments. (A) Grafting of a left limb blastema onto a right stump yield two ‘‘supernumerary limbs’’. Blue and red represent limb tissue with anterior and posterior identity, respectively. (B) Generation of a circular limb then amputation shows that the regenerate always regenerates distal elements from the level of amputation [5].
The role of nerves is crucial for successful regeneration in axolotls, as nerve signals are essential for promoting cell growth and differentiation within the blastema. Disruption of nerve support drastically reduces regenerative capacity, demonstrating the importance of nerve-blastema interactions. The limb regeneration process in axolotls differs from embryonic development and involves unique regulatory mechanisms that ensure the formation of fully integrated and functional structures [4] [5].

Fig. 5 Molecular Dissection of the Supernumerary Limb Phenomenon [5]. (A) In the ‘‘accesory limb model,’’ nerve is re-routed to anterior wound surface, which generates an ‘‘anterior blastema’’ that survives only for 19 days (left). Limb regeneration at the ectopic site is only completed if a piece of posterior limb tissue (red) is grafted to the anterior wound site (right). (B) Provision of SHH to anterior blastemas or FGF8 to posterior blastemas is sufficient for completion of limb regeneration. (C) Molecular circuitry in the regenerating limb blastema established by 12 days of regeneration. Posterior SHH maintains anterior FGF8 expression and vice versa. (D) The FGF8-SHH crosstalk is induced at three sites after left-to-right blastema transplantation (as in Figure 1A), leading to supernumerary limb formation.
Insects: Limb Regeneration and Epigenetic Control
Insects, such as cockroaches and crickets, exhibit remarkable regenerative abilities through a process regulated by three main stages: wound healing, blastema formation, and morphogenesis. Limb regeneration in insects is closely tied to the molting cycle, which allows the remodeling of the exoskeleton to facilitate new limb growth. The initial stage involves the formation of a hemolymph clot to seal the wound, followed by the activation of surrounding epithelial cells, which proliferate to form a blastema [6].
The regenerative process is influenced by physiological factors, such as developmental stage, amputation severity, and hormonal signals. The signaling pathways involved in blastema formation and patterning include epigenetic mechanisms that modulate stem cell behavior, ensuring the correct differentiation and growth of new structures. Molecular analyses have revealed that coordination of patterning and growth is critical for successful regeneration in insects [6].

Fig. 6 Diagram of appendage regeneration in insects [6]. Following injury or amputation, appendage regeneration in nymphal legs (A and B) or larval imaginal discs (C) proceeds through a generally similar process involving three stages: wound healing, blastema formation, and morphogenesis (regenerative growth, differentiation, and patterning). A In some Blattaria and other orders of insects, when amputation is performed at a site proximal to the femur, a new leg can be regenerated under the exoskeleton after one molt. B In some insects of the order Orthoptera, regeneration mainly occurs at the distal site of the tibia. Four molting cycles are needed for the successful regeneration of missing tissue. C After the surgical amputation of the imaginal leg disc, the smaller fragment from the anterior dorsal quadrant regenerates the remainder of the disc, and a new leg can be regenerated after one molt. Co, coxa; Tr, trochanter; Fe, femur; Ti, tibia; Ta, tarsus.
Future Perspectives and Applications in Regenerative Medicine
The regenerative mechanisms observed in animal models provide significant insights for developing advanced regenerative therapies. Activating stem cells, manipulating molecular signals, and modulating the tissue environment could be key tools for promoting regeneration in mammals. Discoveries in animal models could translate into therapies for treating degenerative diseases, injuries, and slowing the aging process. However, applying these insights to human medicine requires a deeper understanding of the mechanisms limiting regeneration in mammals.
Conclusion
The analysis of regenerative models, from planarians to axolotls, has highlighted the complexity of the cellular and molecular processes that govern regeneration. Regenerative capacity depends on a delicate interaction between molecular signals, stem cells, and the tissue environment, coordinating the reconstruction of complex tissues. Understanding these processes offers new perspectives for regenerative medicine and could revolutionize the treatment of degenerative diseases and injuries. Despite the challenges, scientific discoveries continue to shed new light on the regenerative potential of the human body.
References
1. Wenemoser D, Reddien PW. Planarian regeneration involves distinct stem cell responses to wounds and tissue absence. Dev Biol. 2010 Aug 15;344(2):979-91. doi: 10.1016/ j.ydbio.2010.06.017. Epub 2010 Jun 19. PMID: 20599901; PMCID: PMC2950745.
3. Klapper W, Kühne K, Singh KK, Heidorn K, Parwaresch R, Krupp G. Longevity of lobsters is linked to ubiquitous telomerase expression. FEBS Lett. 1998 Nov 13;439(1-2):143-6. doi: 10.1016/s0014-5793(98)01357-x. PMID: 9849895.
5. Tanaka EM. The Molecular and Cellular Choreography of Appendage Regeneration. Cell. 2016 Jun 16;165(7):1598-1608. doi: 10.1016/j.cell.2016.05.038. PMID: 27315477.







No comments