Discover Related Products
Now explore supplements designed to support Longevity, Gut Health & Cellular Vitality.
CITOZYM - Support Natural Detox, Immune and Longevity Support
The Power of Gut Microbiota
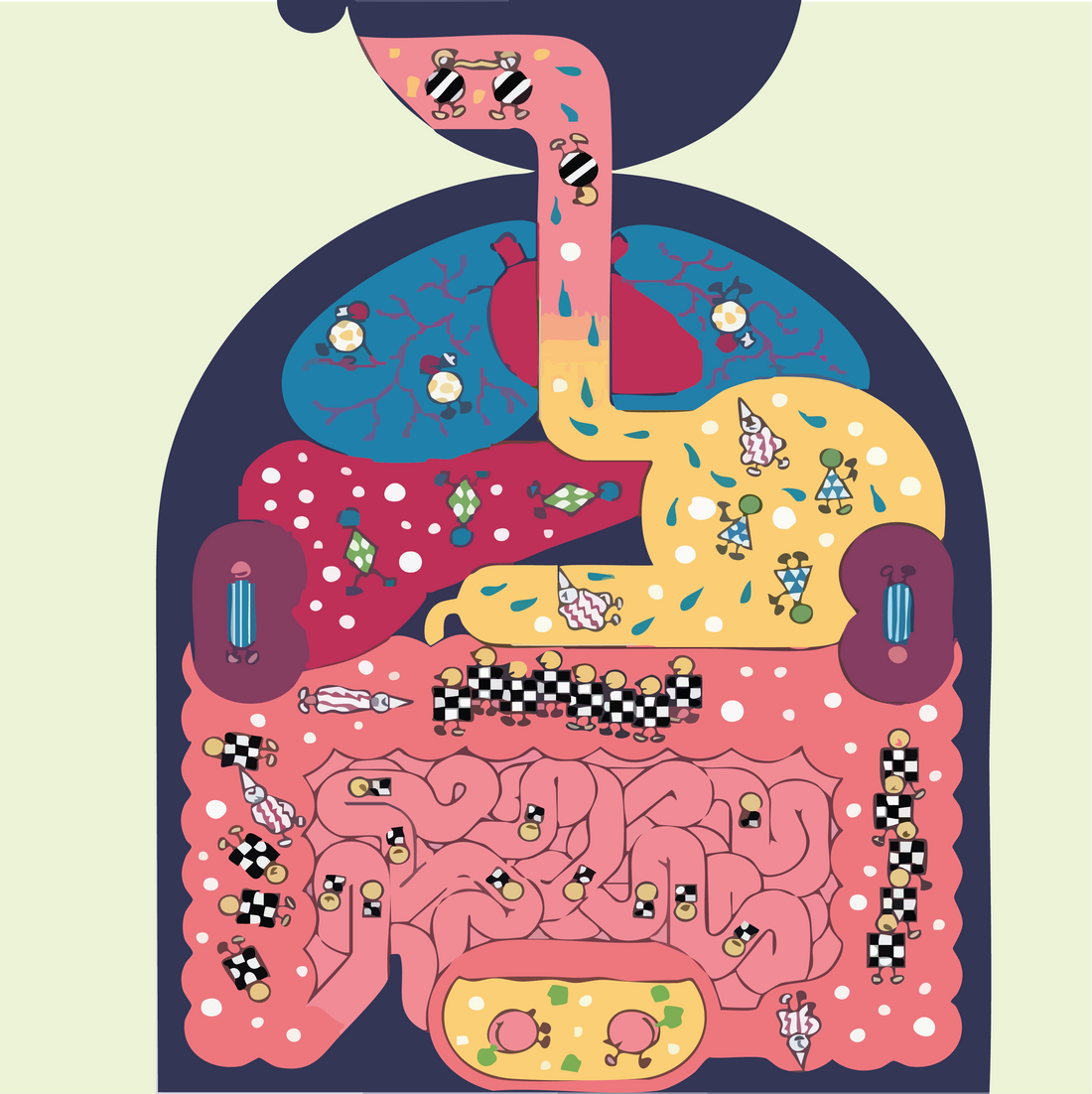
How Nutrition Boosts Immunity, Reduces Inflammation, and Supports Cancer Therapy
Introduction
The gut microbiota, a vast and intricate community of microorganisms residing in the gastrointestinal tract, plays a fundamental role in regulating many of the body’s critical systems, including immune responses, metabolism, and even brain function. Comprising trillions of bacteria, viruses, fungi, and other microbes, this ecosystem is in constant interaction with the host, influencing various physiological processes such as digestion, immune defense, and even mental health [1,2].

Figure 1. Microbiota load and diversity vary throughout the gastrointestinal tract. In a healthy individual, the microbiota count, measured as colony-forming units (cfu)/mL, in the human gastrointestinal tract increases from the stomach/duodenum to the jejunum/ileum to the colon. Additionally, the regional diversity in the gastrointestinal microbiome, known as the microbial landscape, increases from the mouth (rostral) to the anus (caudal). Importantly, there is significant intra- and interpersonal variation in the composition of the human microbiome, which is further influenced by various factors such as the mode of infant delivery and feeding, aging, diet composition, geography, medication, stress, and many others. [3]
The composition of the gut microbiota is shaped by several factors, including diet, lifestyle, medications, and genetics. Disruption of this balance, known as dysbiosis, has been associated with a wide range of chronic diseases, including inflammatory bowel disease (IBD), metabolic disorders, obesity, cancer, and neuropsychiatric conditions [3,4]. This article explores the intricate relationship between diet and gut microbiota, emphasizing the role of probiotics, prebiotics, and other interventions in regulating inflammation, enhancing immune function, and supporting cancer therapies.

Figure 2. Organ dysfunction as a consequence of dysbiosis. The balance of the physiological microbiota community (eubiosis) in the gut is crucial to ensure an individual’s health. Factors such as an unhealthy diet, excessive fasting, alcohol consumption, smoking, physical or psychological stress, chronic inflammation, and the overuse of antibiotics can lead to dysbiosis, which can negatively impact the health of organs. Diseases associated with abnormalities and imbalances in the gut microbiota can vary greatly and affect all organs. The abbreviations used are as follows: ccRCC, clear cell renal cell carcinoma; CVD, cardiovascular disease; HCC, hepatocellular carcinoma; IBD, inflammatory bowel disease; MASH, metabolic-associated steatohepatitis; MASLD, metabolic dysfunction-associated steatotic liver disease; PCOS, polycystic ovary syndrome. [3]
Gut Microbiota: Central Roles, Functions, and Health Impacts
The gut microbiota acts as a “biological shield,” serving as the first line of defense against pathogens while playing a crucial role in immune regulation [1]. This microbial community performs essential functions such as the fermentation of dietary fibers to produce short-chain fatty acids (SCFAs), like butyrate, propionate, and acetate. These SCFAs are critical for maintaining the health of the gut by reinforcing the intestinal barrier, regulating the immune system, and reducing inflammation throughout the body [1,2].
Butyrate, in particular, serves as an energy source for intestinal epithelial cells and has potent anti-inflammatory properties. It inhibits histone deacetylases (HDACs), enzymes involved in the regulation of gene expression, and promotes the differentiation of regulatory T cells (Tregs) that help maintain immune tolerance and prevent autoimmune reactions [4,5]. This regulation is especially significant in inflammatory bowel disease, where butyrate deficiency is associated with exacerbated disease symptoms and greater intestinal inflammation [2].
In addition to its role in inflammation regulation, the gut microbiota maintains the integrity of the intestinal barrier. A balanced microbiota prevents the development of “leaky gut,” a condition where the intestinal barrier becomes permeable, allowing harmful substances such as toxins and antigens to pass into the bloodstream. Increased intestinal permeability can trigger systemic inflammation, which has been linked to conditions such as IBD, metabolic syndrome, and autoimmune disorders [2,5]. Dysbiosis, characterized by a reduction in beneficial bacteria like Bifidobacterium and an increase in harmful strains like Clostridium difficile, contributes to intestinal barrier dysfunction and chronic inflammatory diseases [1,3].
The gut microbiota also interacts with various immune cells, influencing their maturation and activation. For instance, SCFAs can induce the production of cytokines, chemokines, and other signaling molecules that play a crucial role in the immune response [2]. These interactions highlight the microbiota’s pivotal role in shaping both local and systemic immune responses, which are essential for the prevention of infections and inflammation-related diseases.

Figure 3. Immune mechanisms for limiting the commensals within the epithelial layer. Innate immunity: (1) mucin glycoproteins of the gob- let cells form a layer over the epithelia to restrict bacterial adhesion; (2) constitutive expression of α-defensins from epithelial cells with- out microbial signal; (3_ β-defensin expression by muramyl dipeptide of Gram positive bacteria mediated by cytosolic nucleotide-binding oligomerization domain-containing protein 2 (NOD2); (4) C-type lec- tin regenerating islet-derived protein 3γ (REG3γ) via microorganism- associated molecular patterns (MAMP) activates toll-like receptors (TLRs) recruiting cytosolic adaptor myeloid differentiation primary- response protein 88 (MYD88) to restrict bacterial penetration through epithelial layer; (5) dendritic cells (DC) located beneath the epithelial dome of Peyer’s patches take up bacteria and migrate to mesenteric lymph node and induce B cells to differentiate into IgA+ plasma cells for secretion of IgA. Transcytosis of IgA across the epithelial cell layer limits bacterial association with the epithelium. Adaptive immunity: transcytosis of bacteria through M cells and luminal antigen activates DC in the Peyer′s patch and helps to differentiate the naive CD4+ T cells into Treg and TR1 characterized by expression of anti-inflammatory cytokines transforming growth factor (TGFβ) and IL10. The TH1, TH2, and TH17 cells are characterized by proinflammatory cytokines including (1) IFNγ, (2) IL-4, 5 and 13 and (3) IL-23 and IL-17F, respectively. [2]
The Influence of Diet on Gut Microbiota Composition and Function
Diet is a key factor in shaping the composition and functionality of the gut microbiota. The nutrients consumed through the diet influence which microbial species dominate and how they function. Western diets, typically high in saturated fats, refined sugars, and processed foods, are associated with an increased risk of dysbiosis and chronic inflammation [1,2]. This type of diet promotes the growth of pro-inflammatory bacteria while reducing microbial diversity, leading to an imbalance that compromises the microbiota’s ability to regulate immune responses effectively [2].
Fiber-rich diets, such as the Mediterranean diet, support the growth of beneficial bacteria, particularly Bifidobacteriumand Lactobacillus, which ferment dietary fibers to produce SCFAs like butyrate and propionate [1,5]. These SCFAs contribute to gut health by maintaining the integrity of the intestinal barrier and modulating immune responses. Studies have shown that individuals who consume high-fiber diets experience lower levels of systemic inflammation, reducing their risk of chronic conditions such as obesity, type 2 diabetes, and cardiovascular diseases [2,5].
Moreover, the type of fats in the diet influences the microbiota’s composition. Diets high in polyunsaturated fatty acids (PUFAs), such as those found in fish and nuts, have been shown to promote a healthy gut microbiota and reduce inflammation [3]. In contrast, diets rich in saturated fats, commonly found in processed meats and fried foods, are associated with increased intestinal permeability, higher levels of inflammatory markers, and greater microbial dysbiosis [2].

Figure 4. Pro and anti-inflammatory effects of dietary fats. Dietary fats directly and indirectly act as both pro-inflammatory and anti-inflammatory mediators. Saturated fatty acids are pro- inflammatory in nature, through increased translocation and activation of LPS leading increased TLR4 signalling. n-3 PUFAs may be immunosuppressant through its effects on immune cells and intestinal barrier integrity. n-6 PUFAs are mainly pro-inflammatory, however can also produce anti-inflammatory eicosanoids. The ratio of n-6/n-3 PUFAs are important in determining the inflammatory state in the body. Red: pro-inflammatory; Green: anti-inflammatory; Orange: pro- & anti-inflammatory. AA, arachidonic acid; PUFA, polyunsaturated fatty acid; MUFA, monounsaturated fatty acids; LA, linoleic acid; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; COX, cyclooxygenase; LOX, lipoxygenase; PG, prostaglandin; LX, lipoxin. [1]
Polyphenols, a group of bioactive compounds found in fruits, vegetables, tea, and wine, also play a significant role in modulating the gut microbiota. These compounds are not fully absorbed in the small intestine and reach the colon, where they are metabolized by gut bacteria into bioactive compounds with anti-inflammatory and antioxidant properties [5]. Research has demonstrated that polyphenol-rich diets enhance microbial diversity and promote the growth of beneficial bacteria, contributing to improved gut health and reduced inflammation [5].

Figure 5. Bioactive compounds produced by gut microbiota. 1) Dietary bres are fermented by gut microbiota to produce short-chain fatty acids (SCFAs), 2) B vitamins may be produced from gut microbiota metabolism. 3) Amino acids from dietary proteins may be fermented to produce branched-chain fatty acids (BCFA), ammonia, phenols, and hydrogen sul de. 4) Dietary tryptophan is metabolised by gut microbiota to indoles. Pro-inflammatory compounds represented in red, anti-inflammatory represented in green. These compounds affect host physiology via interactions with gut microbiota, colonic epithelial cells and mucosal immune cells. ROS: reactive oxygen species, MAIT, mucosal-associated invariant T cell. [1]
Amino acids, particularly tryptophan, are another dietary component that influences the gut microbiota. Tryptophan is metabolized by gut bacteria into indole derivatives that activate the aryl hydrocarbon receptor (AhR), a key regulator of immune responses [5]. This interaction helps control inflammation in the gut, ensuring a balanced immune response. Deficiency or altered metabolism of tryptophan has been linked to increased susceptibility to inflammatory bowel diseases and other chronic inflammatory conditions [5].

Figure 6. Immune modulating compounds contained within Fermented foods. Bioactive compounds from fermented foods that have anti-inflammatory effects on the activity and phenotype of innate (macrophages, neutrophils, mast cells) and adaptive (T-cells) immune cells. GABA, gamma-aminobutyric acid. [6]
Probiotics and Prebiotics: Key Tools for Modulating Gut Health
Probiotics, defined as live microorganisms that provide health benefits when consumed in adequate amounts, play a crucial role in restoring balance to the gut microbiota, particularly in cases of dysbiosis [3]. Probiotic strains such as Lactobacillus and Bifidobacterium have been extensively studied for their ability to improve gut health by outcompeting harmful bacteria, enhancing the production of SCFAs, and strengthening the gut barrier [3]. These probiotics also modulate immune responses by influencing the production of cytokines, which are key regulators of inflammation.
Research has shown that probiotic supplementation can alleviate symptoms of irritable bowel syndrome (IBS), improve immune function in patients with inflammatory bowel disease (IBD), and restore microbial balance following antibiotic treatment [3,4]. Probiotics have also been investigated for their role in enhancing the gut-brain axis, with studies suggesting that certain strains may help reduce symptoms of anxiety and depression by modulating the production of neurotransmitters and reducing inflammation [4].
Prebiotics, on the other hand, are non-digestible food components that selectively promote the growth of beneficial bacteria in the gut. Fructo-oligosaccharides (FOS) and galacto- oligosaccharides (GOS) are two of the most well-known prebiotics, supporting the growth of bacteria such as Bifidobacterium that produce SCFAs [4]. These prebiotics are fermented in the colon, where they enhance the production of SCFAs and improve gut barrier function, leading to reduced inflammation and improved gut health [4].
Combining probiotics and prebiotics—known as synbiotics—provides a synergistic approach to improving gut health and reducing inflammation [3]. Synbiotics have been shown to be particularly effective in preventing and managing chronic inflammatory conditions, including IBD, by enhancing microbial diversity, promoting the growth of beneficial bacteria, and supporting immune function [3].

Figure 7. Applications of probiotics in the therapy of human diseases. The manipulation of the gut microbiota may be beneficial in treating diseases of the heart, skin, brain, and lung through what are known as interorganic axes (e.g., gut–heart axis, gut–skin axis, gut–brain axis, and gut–lung axis). The diseases listed are just a few examples where probiotics have shown benefits. Additionally, they have a positive impact on general metabolism, making them useful in the treatment of type 2 diabetes, obesity, and cancer, likely by addressing mitochondrial dysfunction. [3]
Gut Microbiota and the Gut-Brain Axis: Implications for Mental Health
The gut-brain axis refers to the bidirectional communication system between the gut microbiota and the central nervous system, involving neural, hormonal, and immune pathways [4]. This communication is critical for maintaining both gut and brain health. Gut bacteria produce a range of metabolites, including SCFAs, serotonin, dopamine, and gamma-aminobutyric acid (GABA), which influence brain function and behavior [4].
Recent research has highlighted the role of the gut microbiota in mental health, with dysbiosis being linked to conditions such as anxiety, depression, and autism spectrum disorders [4]. Dysbiosis can alter the production of key neurotransmitters and increase gut permeability, allowing inflammatory molecules to enter the bloodstream and reach the brain, contributing to neuroinflammation and cognitive dysfunction [4]. This connection underscores the importance of maintaining a healthy gut microbiota for mental well-being.
Probiotics, particularly those containing strains of Lactobacillus and Bifidobacterium, have shown promise in modulating the gut-brain axis and reducing symptoms of anxiety and depression [4]. These probiotics may influence the production of neurotransmitters and modulate inflammatory pathways, highlighting their potential as a therapeutic option for improving mental health [4].

Figure 8. The communication pathway of the gut–brain axis in a depression patient. The HPA axis is activated under internal or external pressure. The hypothalamus activates the release of CRH, promotes the release of ACTH by the pituitary gland, and results in the release of cortisol by the adrenal gland. Cortisol is a hormone that affects intestinal integrity, motility, and mucus production, inducing changes in the composition of intestinal microbiota. In patients with depression, this pathway is significantly enhanced and HPA axis activity is overactive. The end result is often IBS and disrupted gut microbiota. Microbial metabolites including short-chain fatty acids and microbial neural substrates such as catecholamines, histamines, and GABA can act on intestinal epithelial cells and stimulate intestinal nerves, which will stimulate the central nervous system through the vagus nerve. Some SCFAs and peptide can cross the blood–brain barrier and act directly on the central nervous system. LPS on the surface of Gram-negative bacteria can mediate the effects of immune cells and the vagus nerve on the brain. HPA, hypothalamus– pituitary–adrenal; CRH, corticotropin-releasing hormone; ACTH, adrenocorticotropic hormone; CNS, central nervous system; GABA, γ-aminobutyric acid; SCFA, short-chain fatty acid; LPS, lipopolysaccharide; IBS, irritable bowel syndrome. [4]
The Role of Gut Microbiota in Cancer Therapy: A New Frontier
Emerging evidence suggests that the gut microbiota plays a crucial role in modulating the efficacy of cancer therapies, particularly immune checkpoint inhibitors (ICIs) [6]. These therapies work by enhancing the immune system’s ability to target and destroy cancer cells. However, the composition of the gut microbiota can significantly influence patient outcomes, with those possessing a more diverse microbiota showing better responses to ICIs compared to those with dysbiosis [6].
Specific bacterial strains, such as Akkermansia muciniphila and Bifidobacterium longum, have been associated with improved responses to immunotherapy, likely due to their ability to enhance immune activation and promote T cell responses [6]. These bacteria may also help reduce the adverse effects of cancer therapies by modulating immune function and maintaining the integrity of the gut barrier [6].
In addition to influencing immunotherapy outcomes, the gut microbiota may also play a role in modulating chemotherapy efficacy. Dysbiosis has been linked to reduced chemotherapy effectiveness and increased treatment-related toxicity [6]. Preclinical studies have shown that modulating the microbiota through prebiotics, probiotics, or fecal microbiota transplants (FMT) can enhance chemotherapy responses and reduce side effects [6].
As research continues to uncover the links between the gut microbiota and cancer therapy, new therapeutic strategies are emerging that involve modulating the microbiota to improve treatment outcomes and enhance patient well-being. These findings underscore the potential of gut- targeted interventions as a valuable addition to existing cancer therapies.

Figure 9. Tumor Immunosurveillance. Tumor immunosurveillance can be divided into two parts, namely innate immunity and adaptive immunity. The former involves various types of myeloid lineage cells and innate lymphoid cells (ILCs), such as macrophage and NK cell. NK cells can kill tumor cells through antibody-dependent cell-mediated cytotoxicity (ADCC), FAS-FASL pathways and perforin-granzyme B. In addition to ADCC and opsonization, macrophages also act as antigen-presenting cells (APC). Adaptive immunity begins with tumor antigen recognized by T cell receptor (TCR), during which dendritic cells (DCs) play a dominant role. Neoantigens generated during oncogenesis are released and captured by DCs for processing. DCs present antigen peptide to T cells in the form of peptide-MHC complex (pMHC). TCR-pMHC interaction combined with costimulatory signal results in the priming of effector T cells. Then the activated T cells, which can specifically target the cancer cells, migrate to the tumor bed and kill the cancer cells through direct cytotoxic effect or producing cytokines to recruit more immune cells. [6]
Conclusion
The relationship between diet, gut microbiota, and immune function is crucial for maintaining overall health and preventing a wide range of chronic diseases. The composition of the gut microbiota is strongly influenced by dietary factors, and interventions such as probiotics, prebiotics, and synbiotics offer promising strategies for modulating the microbiota to support immune health, reduce inflammation, and improve mental and physical well-being. As research continues to shed light on the intricate connections between the gut microbiota, immune system, and disease, personalized therapeutic approaches targeting the microbiota hold great potential for improving health outcomes in conditions ranging from inflammatory bowel disease to cancer.
References
-
Gill PA, Inniss S, Kumagai T, Rahman FZ, Smith AM. The role of diet and gut microbiota in regulating gastrointestinal and inflammatory disease. Front Immunol. 2022 Apr 5;13:866059.
-
Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76:473-493.
-
Chandrasekaran P, Weiskirchen S, Weiskirchen R. Effects of probiotics on gut microbiota: An overview. Int J Mol Sci. 2024 May 30;25(6022):6022.
-
Zhu F, Tu H, Chen T. The microbiota–gut–brain axis in depression: The potential pathophysiological mechanisms and microbiota combined antidepression effect. Nutrients. 2022;14(2081):2081.
-
Fan L, Xia Y, Wang Y, et al. Gut microbiota bridges dietary nutrients and host immunity. Sci China Life Sci. 2023 Nov;66(11):2466-2514.
-
Liu X, Chen Y, Zhang S, Dong L. Gut microbiota-mediated immunomodulation in tumor. J Exp Clin Cancer Res. 2021;40:221.






No comments